Modeling organogenesis from biological first principles
Organization in biology
Here we discuss the application and articulation of biological principles for mathematical modeling of morphogenesis in the case of mammary ductal morphogenesis, with an emphasis on the default state.
Abstract
Unlike inert objects, organisms and their cells have the ability to initiate activity by themselves, and thus change their properties or states even in the absence of an external cause. This crucial difference led us to search for principles suitable for the study organisms. We propose that cells follow the default state of proliferation with variation and motility, a principle of biological inertia. This means that in the presence of sufficient nutrients, cells will express their default state. We also propose a principle of variation that addresses two central features of organisms, variation and historicity. To address interdependence between parts, we use a third principle, the principle of organization: more specifically, the notion of the closure of constraints. Within this theoretical framework, constraints are specific theoretical entities defined by their relative stability with respect to the processes they constrain. Constraints are mutually dependent in an organized system and act on the default state.
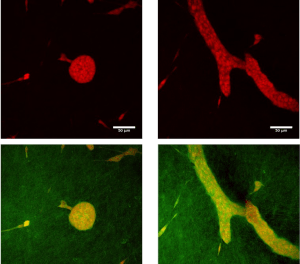
Here we discuss the application and articulation of these principles for mathematical modeling of morphogenesis in a specific case, that of mammary ductal morphogenesis, with an emphasis on the default state. Our model has both a biological component, the cells, and a physical component, the matrix that contains collagen fibers. Cells are agents that move and proliferate unless constrained; they exert mechanical forces that i) act on collagen fibers and ii) on other cells. As fibers are organized, they constrain the cells’ ability to move and to proliferate. This model exhibits a circularity that can be interpreted in terms of the closure of constraints. Implementing our mathematical model shows that constraints to the default state are sufficient to explain the formation of mammary epithelial structures. Finally, the success of this modeling effort suggests a step-wise approach whereby additional constraints imposed by the tissue and the organism can be examined in silico and rigorously tested by in vitro and in vivo experiments, in accordance with the organicist perspective we embrace.